About NEOMUNE
The overall NEOMUNE objective is to develop new diet and gut microbiota treatments for newborn infants.
Shortly after birth, infants must adapt to milk intake and tolerate colonization by billions of microbes along all body surfaces, including the gut. Together, the first diet and gut microbiota stimulate immunity and digestive development. Most newborn infants normally adapt well, but factors such as Caesarean birth, high hygiene levels, antibiotics treatment and inappropriate formula feeding may disturb immune system maturation.
Impaired immune system maturation is associated with reduced growth, more infections and impaired maturation of key organs like the gut and brain.
The most sensitive population and at greatest risk of an improper maturation after birth is infants born small or premature, and these account for approximately 15% of all infants. It remains unknown how diet and bacterial colonization best support maturation for term, preterm and low birth weight infants.
The overall NEOMUNE objective, to develop new diet and gut microbiota treatments for newborn infants, is divided into five sub-objectives
- To demonstrate if differences in the gut microbiota shortly after birth, is related to later infection resistance, gut and brain maturation.
- To test if different milk feeding regimens will affect immunity, gut and brain maturation, directly or via changes to the gut microbiota.
- To provide proof of concept for the use of novel milk diets, milk-derived components, or probiotic bacterial cultures in newborn infants.
- To lower the societal burden of infants (term, preterm) that show poor growth, organ immaturity and low infection resistance, both short and long term.
- To identify the cultural factors that affect clinical care procedures for weak newborn infants in different countries, and show how these influence novel methods.
The objectives will be studies in three work packages.
- The neonatal period: A ‘window’of maturational opportinity
- Hygiene hypothesis
- Effects of early diet on gut and brain maturation
- Type of early diet
The neonatal period: A ‘window’ of maturational opportinity
At birth, there is a dramatic shift from a sterile environment with constant placental supply of nutrients, to a microbe-rich environment with intermittent uptake of complex milk nutrients via the gut. A successful birth transition involves rapid mucosal maturation and tolerance to food antigens and the billions of colonizing microbes during the first days and weeks (1).
Newborns depend mainly on inborn (innate) immunity, while adaptive, cell-mediated immunity gradually develops throughout the body. Maturation of immunity interacts with development of many organ systems, and is influenced by factors in milk and by colonizing bacteria just after birth. The neonatal period thus becomes a highly sensitive ‘window’ of maturation that influences health at this time, but also later in life (2-3).
Hygiene hypothesis
This forms the background for our overall working hypothesis in NEOMUNE:
Optimal milk and microbiota in early life improve later immune, gut and brain functions
Immune-related disorders have increased over the last 50 years in industrialized countries and have led to the so-called 'hygiene hypothesis'. This hypothesis suggests that exposure to a balanced microbiota early in life, with a well-controlled activation of Toll-like receptors (TLRs) on epithelial and immune cells, is crucial in facilitating optimal immune maturation (2-6).
The evidence for the hygiene hypothesis remains weak and is based mainly on population studies on allergies and other auto-immune diseases in older children and adults. Few studies have addressed the important question of how we can best support development immediately after birth, and provide optimal resistance against both mucosal and systemic infections.
Effects of early diet on gut and brain maturation
Cross-talk among immune cells in different parts of the body connects the local gut mucosal immunity with systemic immunity (2) and with immunity at more distant sites such as the brain (7). In the neonatal period, the gut and the brain are both very sensitive to inflammatory insults and infections (1-3,7-8). Milk and microbiota interventions may affect gut and brain functions by affecting the inter-organ, neuro-endocrine signaling, and by the circulating gut-derived inflammatory mediators, intact milk immunomodulatory factors and metabolites of dietary or bacterial origin (7-10).
The phenotype, density and cytokines of the brain glial cells may play the key role for brain functions that are affected by diet and microbiota (10-12). Such combined diet and microbiota effects on the gut and brain are likely to be most pronounced in hyper-sensitive newborns, such as preterm or growth-restricted infants (born <37 weeks gestation, <2000 g, 15% of all infants) (8,13-15).
Due to immaturity, such infants are particularly dependent on milk bioactive factors and a balanced microbiota, but they are also intolerant to large amounts of oral feeds (8,13), leading to ‘minimal enteral nutrition’ (MEN) for the first week(s).
Brain dysfunction is the most serious late complication of preterm birth and is more prevalent after the feeding and bacteria-dependent gut-inflammatory disease, necrotizing enterocolitis (NEC) (8). The links may be direct or indirect, but the recent finding that gut microbial colonization affects brain maturation and motoric control in mouse pups (10) supports direct links among diet, immunity, gut and brain in early life. In both preterm and term infants, breastfeeding relates to fewer infections (16) and improved gut health and cognition (13,14).
Birth by Caesarean section in high-hygiene hospital environments, and widespread use of antibiotics, are factors that reduce gut microbiota density and diversity in the newborn for some time after birth (5,20-22). On the other hand, high-hygiene environments and antibiotics are essential tools to combat infections, especially for the weakest newborn infants.
Type of early diet
The time is now ripe for a concerted effort that verifies early diet and gut colonization effects on maturation of immunity gut and brain.
It remains unknown, how the effects of milk and colostrum, from own mother, other mothers, or even from another species, exert immuno-modulatory effects. There is some evidence from new animal models (17-19) but more studies, from basic mechanisms to clinical application, are required.
Knowledge of diet and microbial factors that influence immune maturation is crucial in designing milk formulas for infants for whom breastfeeding is restricted, or not possible. Addition of probiotics to milk feedings have been speculated to promote colonization of beneficial gut bacteria, suppress pathogens and stimulate immune development (19,23,24), but the current level of evidence inhibits widespread use of probiotics, especially for vulnerable newborn infants. Likewise, intense focus is also on the prebiotic human milk oligosaccharides (HMOs) that may promote epithelial defense and healthy gut colonization (24,25), but effects in newborns are not known.
The benefits of breast-feeding may be explained by absorption of milk bioactives, such as milk fat globule membrane (MFGM) fractions, polyunsaturated fatty acids (PUFA), caseinoglycomacropeptide (CGMP) and phospholipids (PL) and preliminary evidence suggests effects on immunity, gut and brain (13,14,26,27). Beneficial effects may even be induced by feeding amniotic fluid (‘the fetal enteral diet’), as indicated in studies in newborn pig (18) and mice (28).
New advice for diet and microbiota regimens for infants must be based on safety, science and technical and commercial availability of high-quality products.
References
- Sangild PT (2006). Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med 231,1695
- Levy O (2007). Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature Reviews Immunol 7,379.
- Calder et al. (2006). Early nutrition and immunity: progress and perspectives. Br J Nutr 96,774.
- Fink et al. (2012). Establishment of tolerance to commensal bacteria requires a complex microbiota and is accompanied by decreased chemokine expression. Am J Physiol 302,G55.
- Neu et al. (2011). Cesarean versus vaginal delivery: long term outcomes and the hygiene hypothesis. Clin Perinatol 38,321.
- Moulder et al. (2009). Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces BMC Biology 7,79.
- Hagberg et al. (2012). Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 71,444.
- Martin et al. (2010). Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr. 157,751.
- Harrie et al. (2008). Nutritional factors influence infections in preterm infants. J Nutr 138,1813S.
- Heijtz et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 15,108:3047.
- Dilger R and Johnson R (2008). Aging, microglial cell priming, and the discordan central inflammatory response to signals from the peripheral immune system. J Leukoc Biol 84,932.
- Conrad et al. (2012). Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: A longitudinal MRI study. Dev Neurosci (in press).
- Lucas et al. (1998). Randomised trial of early diet in preterm babies and later intelligence quotient Br Med J 317,1481.
- Isaacs et al. (2009). Early diet and general cognitive outcome at adolescence in children born at or below 30 weeks gestation. J Pediatr 155,229.
- Kieviet et al. (2012). Brain development of very preterm and very low-birth weight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol 54,313.
- Duijts et al. (2009). Breastfeeding protects against infectious diseases during infancy in industrialized countries. A systematic review. Matern Child Nutr 5,199-210.
- Sangild et al. (2006). Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130,1776.
- Siggers et al. (2011). Nutritional modulation of the gut microbiota and immune system in preterm neonates susceptible to necrotizing enterocolitis. J Nutr Biochem 22,511.
- Cilieborg et al. (2012). Bacterial colonization and gut development in preterm neonates. Early Hum Dev 88,S41.
- Hansen et al. (2012). Patterns of early gut colonization shape immune responses. PLoS One.7:e34043.
- Jiang et al. (2012). Antibiotics increases gut metabolism and antioxidation proteins and decreases acute phase response and NEC in preterm neonates. PloS One (in press).
- Cho et al. (2012). Antibiotics in early life alter murine colonic microbiome and adiposity. Nature 30;488,621.
- Deshpande et al. (2011). Progress in the field of probiotics. Curr Opin Gastroenterol 27,13.
- Lafeber et al.(2008). Nutritional factors influence infection in preterm infants. J Nutr 138,1813S
- Jantscher-Krenn E and Bode L (2012). Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr 64,83.
- Mikkelsen et al. (2005). Sialic acid-containing milk proteins show differential immunemodulatory activities independent of sialic acid. J Agric Food Chem 53,7673.
- Tanaka et al. (2012). Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy. Brain Dev (in press).
- Good et al. (2012). Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA, 10,109,11330.
- Quigley et al. (2007). Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 17;4,CD002971
- Bombell S and McGuire W (2009) Early trophic feeding for very low birth weight infants. Cochrane Database Syst Rev 2009 8;3,CD000504
- Nowotny et al. (2001): Re-thinking Science. Knowledge and the Public in an Age of Uncertainty. Cambridge Polity Press
- Latour B. (1987). Science in Action. Harvard University Press.
- Ellen RF (1984). Ethnographic research. A Guide to General Conduct. London: Academic Press.
- Kirkeby OF (2006). Det nye lederskab, Børsens Forlag, 280 pp. København (Danish).
Optimal care for newborn infants, including those born premature, growth restricted or otherwise compromised, is a key objective in all modern societies. As such, NEOMUNE targets a central society-relevant topic. Due to infant, maternal, societal and cultural factors, there is great variability in maternal care and feeding practices. It is important to provide evidence for best clinical practice for both normal and compromised newborn infants.
The current level of evidence allows for only few universally-accepted clinical care procedures for infants with limited maternal care. We are aware of the great complexity in targeting immune, gut and brain functions, and in both preterm and term newborn infants. On the other hand, this combined target is key to the novelty of the NEOMUNE platform. The combined target is also consistent with the health claims already widely used by the infant formula industry to market specialized formulas.
We seek translation of the NEOMUNE results into sustainable solutions for infants. While all results may not have immediate commercial value, we expect them to lead to scientific and clinical advances. Key players in the infant formula industry will have access to proof of concept for new specialized infant formula products, and to test how the infant gut microbiota can be manipulated.
Novel animal models will be designed to be idea-generating and to provide mechanistic scientific support for new interventions in infants. In addition to the pre-determined protocols, the platform will allow room for a limited number of pilot experiments with novel diets and ingredients.
Social science is included in NEOMUNE to better know the potentials and limitations of the new results. Optimal infant care depends on science, but also on social, cultural and ethical understandings of newborn infants and their parents. These vary in different parts of the world, partly explaining the wide differences in feeding practices, especially for infants not having normal access to mother´s milk. The social science studies in NEOMUNE helps to reach sustainable health solutions that are sensitive to public opinion, regulations and cultural context of care takers.
NEOMUNE targets newborn infants with special requirements for maternal and clinical care (e.g. compromised infants devoid of breast-feeding). To avoid small sample size in studies, it is important to establish large, international collaborative networks. Additionally, these are important for the overall research quality and effect. The members list shows that NEOMUNE is highly international and involve leading experts in the field.
NEOMUNE includes leading international infant formula companies. It is important that NEOMUNE is open to inputs from different parts of the world to better define potentials and limitations. Treatment practices for newborn infants and their mothers differ among different parts of the world. At the biological level, this is investigated in WP 1.6 by establishing a data base for different procedures and feeding regimens for preterm infants in China, Netherlands and Denmark. The international cultural challenges are also given particular focus also in WP 1.7 where social and cultural differences in clinical infant care between China and Europe are described.
NEOMUNE will strongly encourage exchange of (young) researchers among different study sites and laboratories, facilitating both scientific and cross-cultural learning. The NEOMUNE associated annual PhD course in “Food, Medicine and Philosophy in East and West” underlines the aim to relate NEOMUNE science to the wider global society and health perceptions, beyond those held by hospitals and laboratories.
We encourage NEOMUNE research groups to speak across borders, not only geographical but just as much scientific and cultural scientific borders within the same country, and across the borders between universities, hospitals and industries. Each partner in NEOMUNE has their own traditions and language, but by interacting with neighboring fields we are able to translate basic science into new knowledge that is relevant for society. We illustrate this basic principle in a figure:
Translational Pediatric Research
How to get from bench to bedside – and beyond?
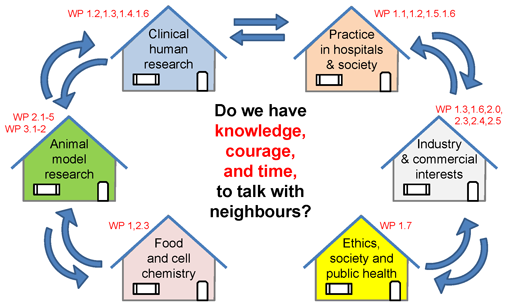
NEOMUNE involves studies in healthy and sick infants. Such research has methodological and ethical limitations. Piglets and mice studies are used to gain information not possible from studies in infants. Data and biological samples are stored, distributed and analysed in many different countries that differ in cultural, clinical and scientific traditions. We work closely with industrial partners with commercial interests that support, but may also conflict with the interests of university and hospital partners.
The highly interdisciplinary and translational research in NEOMUNE creates ethical dilemmas that require careful decisions sensitive to the different cultural contexts of the scientists, parents, medical staff and industry partners. Many scientific aspects are already tightly regulated, exemplified by the required ethical approval to initiate studies in infants and animals, and the role of commercial partners in public research programs.
We encourage NEOMUNE researchers to have open discussions internally and with the public about ethical implications of research in the field. Discussions are facilitated by the inclusion of a separate work package that focusses on the ethical, social and cultural elements of translational research (WP 1.7).
NEOMUNE has no specific ethical views beyond current legislations but acknowledges some ethical dilemmas for the research field, e.g.:
-
Breast-feeding is considered optimal for mother and child, and improved formula composition must not discourage mothers that are able to breast-feed their child.
-
Infant studies must be designed to minimize the risk and discomfort and must balance this against potential benefits to these infants as well as to the infant population.
-
Animal studies must be designed to minimize pain and discomfort but a degree of discomfort for some individuals is difficult to avoid when animals act as a model for sick infants.
-
The care for compromised newborn infants and for experimental animals may be viewed differently in different countries and cultural contexts, precluding a universal ethical standard.
-
While collaboration with industry is important for scientific, societal and financial reasons, it requires careful consideration related to possible bias and conflicts of interest.
-
Cultural and epistemological barriers may reduce translation of results between species (infants, animals), continents (Europe, US, Asia), and organisations (university, hospital, industries).
Supplier of public funding
The Danish Council for Strategic Research
Related projects
The NEOCOL Project
Supplier of public funding
Universities
- University of Copenhagen
- University of Aarhus
- University of Giessen
- Technical University of Denmark
- Sun Yat-sen University Guangzhou
Hospitals
- Copenhagen University Hospital
- Odense University Hospital
- VU Medical Center Amsterdam
- Hospitals in different countries
Industries
- ARLA Foods amba
- ARLA Foods Ingredients
- Biofiber Damino
- Danone Nutricia Research
- Fresenius Kabi

